Topic nitrogen cycle in aquatic ecosystem: Explore the wonders of the nitrogen cycle in aquatic ecosystems, a vital process sustaining life beneath the waves, from tiny microbes to majestic marine fauna.
Table of Content
- What are the microbial transformations involved in the nitrogen cycle in aquatic ecosystems?
- Overview of Nitrogen Cycle Processes
- Importance of Nitrogen in Aquatic Ecosystems
- Nitrogen Fixation: Atmospheric, Industrial, and Biological Methods
- Roles of Microorganisms in the Nitrogen Cycle
- Nitrification: Conversion of Ammonia to Nitrites and Nitrates
- YOUTUBE: The Nitrogen Cycle
- Denitrification: Reverting Nitrates to Nitrogen Gas
- Human Impacts on the Nitrogen Cycle
- Effects of Nitrogen on Aquatic Life
- Managing Nitrogen Levels in Aquatic Ecosystems
What are the microbial transformations involved in the nitrogen cycle in aquatic ecosystems?
In the nitrogen cycle in aquatic ecosystems, there are several microbial transformations that play a crucial role in the conversion of nitrogen into different forms. These transformations include:
- Nitrogen Fixation: Certain microorganisms, such as cyanobacteria and some bacteria, have the ability to convert atmospheric nitrogen (N2) into ammonium (NH4+), which is a form that can be utilized by plants and other organisms.
- Assimilation: Plants and algae take up the ammonium from the water and incorporate it into their tissues to build proteins and other organic compounds. This assimilation by primary producers is an essential step in the nitrogen cycle.
- Nitrification: Nitrification is a two-step process carried out by different groups of bacteria. Firstly, ammonia (NH4+) is oxidized by ammonia-oxidizing bacteria (e.g., Nitrosomonas) to nitrite (NO2-). Secondly, nitrite is further oxidized by nitrite-oxidizing bacteria (e.g., Nitrobacter) into nitrate (NO3-).
- Denitrification: Denitrification occurs in oxygen-limited conditions where certain bacteria (e.g., Pseudomonas, Bacillus) convert nitrate (NO3-) back into atmospheric nitrogen (N2) or nitrous oxide (N2O), releasing it back into the atmosphere. This step is important for the removal of excess nitrogen from aquatic ecosystems.
- Anammox: Anammox (Anaerobic Ammonium Oxidation) is a specialized process conducted by anaerobic bacteria, such as \"Candidatus Brocadia.\" These bacteria can directly oxidize ammonium (NH4+) with nitrite (NO2-) to generate nitrogen gas (N2) without the need for oxygen.
These microbial transformations are interconnected and function in a cyclical manner, allowing nitrogen to be continuously recycled and converted into various forms throughout the aquatic ecosystem. This cycle is essential for maintaining the nitrogen balance and availability within the ecosystem, supporting the growth and development of aquatic organisms.
READ MORE:
Overview of Nitrogen Cycle Processes
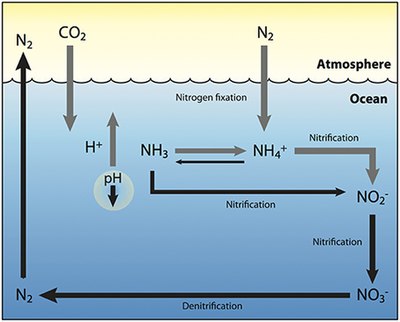
The nitrogen cycle in aquatic ecosystems is a complex process, crucial for sustaining life in water bodies. This cycle describes how nitrogen moves between the atmosphere, aquatic environments, and living organisms, ensuring the availability of essential nutrients for aquatic flora and fauna. The cycle encompasses several key processes:
- Nitrogen Fixation: Atmospheric nitrogen (N2) is converted into ammonia (NH3) or nitrate (NO3-) compounds, making it accessible to living organisms. This can occur through biological fixation by bacteria and cyanobacteria, or through physical processes like lightning.
- Ammonification (Decomposition): Organic nitrogen from dead organisms and waste products is converted back into ammonia by decomposers, such as bacteria and fungi, making it available once more for use in the ecosystem.
- Nitrification: Ammonia is oxidized to nitrites (NO2-) and then to nitrates (NO3-) by nitrifying bacteria. This process makes nitrogen available in a form that can be absorbed by plants.
- Assimilation: Aquatic plants absorb ammonium (NH4+), ammonia (NH3), and nitrate ions through their roots from the water, incorporating these into essential organic compounds like amino acids and proteins.
- Denitrification: The conversion of nitrates back into nitrogen gas (N2), which is released into the atmosphere, is facilitated by denitrifying bacteria. This process completes the nitrogen cycle, allowing nitrogen to exit the aquatic environment and re-enter the atmosphere.
These processes are interconnected, ensuring the continuous recycling of nitrogen, a critical component for life. The balance and efficiency of the nitrogen cycle are vital for the health of aquatic ecosystems, influencing water quality, biodiversity, and the productivity of aquatic environments.

Importance of Nitrogen in Aquatic Ecosystems
Nitrogen plays a pivotal role in the health and productivity of aquatic ecosystems. As a fundamental building block of amino acids, proteins, and nucleic acids, nitrogen is essential for the growth and survival of all aquatic life forms. The importance of nitrogen extends through various facets:
- Supports Plant and Algal Growth: Nitrogen is a key nutrient for aquatic plants and algae, enabling photosynthesis and growth. This, in turn, supports food webs by providing a primary food source for herbivores in aquatic environments.
- Regulates Biodiversity: The availability of nitrogen can influence species composition and biodiversity within aquatic ecosystems. Variations in nitrogen levels can lead to shifts in the types of plants and algae that dominate an area, affecting the entire ecosystem.
- Water Quality: Nitrogen levels are closely linked to water quality. While nitrogen is necessary for aquatic life, excessive amounts can lead to eutrophication, causing harmful algal blooms that deplete oxygen and create dead zones where few organisms can survive.
- Supports Aquatic Life Cycle: By participating in the nitrogen cycle, nitrogen supports the life cycles of various aquatic organisms. It is integral to the food web, from the smallest microorganisms to the largest aquatic animals.
Understanding and managing nitrogen levels in aquatic ecosystems is crucial for maintaining biodiversity, ensuring good water quality, and supporting the livelihoods of communities dependent on these water bodies for food, recreation, and economic activities.
Nitrogen Fixation: Atmospheric, Industrial, and Biological Methods
Nitrogen fixation is a crucial process in the nitrogen cycle, converting atmospheric nitrogen (N2) into a form that organisms can use. This process occurs through several methods, each playing a unique role in aquatic ecosystems:
- Atmospheric Fixation: Lightning strikes convert atmospheric nitrogen into nitrogen oxides (NOx), which dissolve in rainwater and enter aquatic ecosystems as nitrate (NO3-). This natural process contributes a small but significant portion of the nitrogen available in water bodies.
- Industrial Fixation: The Haber-Bosch process synthesizes ammonia (NH3) from atmospheric nitrogen and hydrogen gas under high pressure and temperature, using a catalyst. Although primarily used for agricultural fertilizers, some of this industrially fixed nitrogen can run off into aquatic systems, influencing their nitrogen content.
- Biological Fixation: The most significant source of fixed nitrogen in aquatic ecosystems, biological fixation is carried out by specialized bacteria and cyanobacteria. These organisms possess the enzyme nitrogenase, enabling them to convert atmospheric nitrogen into ammonia. Symbiotic bacteria, such as those associated with the roots of leguminous plants, and free-living bacteria in the soil and water, are key players in this process. In aquatic environments, cyanobacteria are particularly important for fixing nitrogen directly in the water.
Each of these nitrogen fixation methods contributes to the availability of usable nitrogen within aquatic ecosystems, supporting a wide range of life forms from microscopic algae to large aquatic mammals. Understanding these methods is key to appreciating the complexity and importance of the nitrogen cycle in maintaining healthy aquatic environments.
Roles of Microorganisms in the Nitrogen Cycle
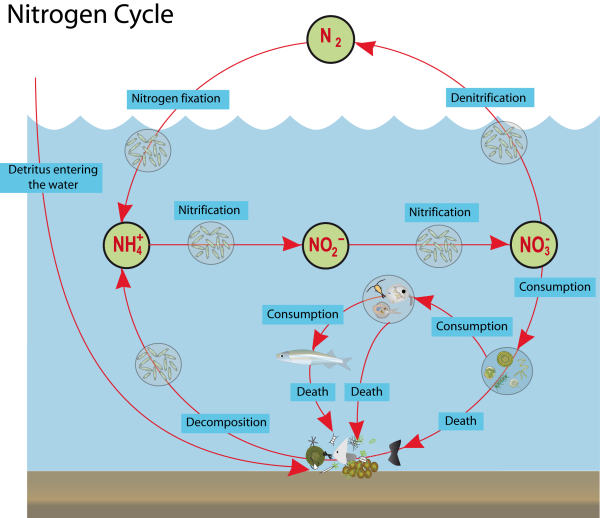
Microorganisms play indispensable roles in the nitrogen cycle, facilitating the conversion of nitrogen among various chemical forms. These tiny yet powerful organisms drive the cycle forward, ensuring the availability of nitrogen essential for aquatic life. Their roles include:
- Nitrogen Fixation: Certain bacteria and cyanobacteria, known as diazotrophs, convert atmospheric nitrogen (N2) into ammonia (NH3), making nitrogen available to living organisms. This process primarily occurs in soil and water, where these microorganisms live.
- Nitrification: A two-step process carried out by different groups of bacteria. First, ammonia is oxidized to nitrite (NO2-) by ammonia-oxidizing bacteria (e.g., Nitrosomonas). Then, nitrite is further oxidized to nitrate (NO3-) by nitrite-oxidizing bacteria (e.g., Nitrobacter). This conversion is crucial for making nitrogen available to plants in a form they can assimilate.
- Denitrification: Denitrifying bacteria, such as Pseudomonas and Clostridium, convert nitrate (NO3-) back into nitrogen gas (N2), releasing it into the atmosphere. This process is vital for maintaining the balance of nitrogen in aquatic ecosystems and preventing the accumulation of excess nitrates.
- Anammox: In anaerobic conditions, anammox bacteria (e.g., Brocadia) oxidize ammonia directly to nitrogen gas, using nitrite as an electron acceptor. This relatively recently discovered pathway is significant in removing nitrogen from aquatic systems.
- Ammonification: Decomposers, including bacteria and fungi, break down organic nitrogen from dead organisms and waste products, converting it back into ammonia. This process recycles nitrogen within the ecosystem, making it available for other organisms to use.
The interactions among these microorganisms ensure the continuous flow of nitrogen through the ecosystem, supporting the growth and survival of a wide array of aquatic life. The health and balance of aquatic environments heavily depend on the efficient functioning of these microbial-driven processes in the nitrogen cycle.
Nitrification: Conversion of Ammonia to Nitrites and Nitrates
Nitrification is a critical step in the nitrogen cycle, primarily occurring in soil and water, where specialized microorganisms convert ammonia into nitrites and then nitrates. This two-stage aerobic process is essential for making nitrogen available to plants in a form they can readily absorb. The stages of nitrification include:
- Ammonia Oxidation: In the first step, ammonia (NH3) is oxidized to nitrite (NO2-) by ammonia-oxidizing bacteria (AOB), such as Nitrosomonas. This conversion requires oxygen, making it an aerobic process.
- Nitrite Oxidation: Following ammonia oxidation, nitrite-oxidizing bacteria (NOB), such as Nitrobacter, further oxidize nitrite to nitrate (NO3-). Nitrates are the end product of nitrification and the form of nitrogen most commonly utilized by plants.
Both steps of nitrification are carried out by distinct groups of autotrophic bacteria, which derive energy from these conversions. The process is crucial for the health of aquatic ecosystems, as it recycles ammonia, produced by decomposition of organic matter, into nitrates, preventing the buildup of toxic ammonia levels and ensuring the availability of nitrogen for aquatic plants and algae.
- Environmental Significance: Nitrification plays a key role in controlling nitrogen levels in water bodies, impacting water quality and the overall health of aquatic ecosystems. It is also a vital process in wastewater treatment, helping to remove excess nitrogen from water before it is released back into the environment.
Understanding nitrification is essential for managing aquatic environments, as imbalances in this process can lead to eutrophication, harmful algal blooms, and oxygen depletion in water bodies.

The Nitrogen Cycle
Step into the mesmerizing world of ecosystems and discover the delicate balance of nature. This captivating video takes you on a journey through lush forests, vibrant coral reefs, and vast grasslands, showcasing the incredible diversity of life thriving within these interconnected habitats.
Nitrogen in the Ocean
Dive into the depths of the ocean and behold its breathtaking wonders. From the enchanting dance of colorful fish to the graceful movements of majestic whales, this awe-inspiring video transports you to a world teeming with fascinating marine creatures and unexplored mysteries.
Denitrification: Reverting Nitrates to Nitrogen Gas
Denitrification is a crucial biological process in the nitrogen cycle, where nitrates (NO3-) are converted back into nitrogen gas (N2), effectively removing bioavailable nitrogen from aquatic ecosystems. This anaerobic process is performed by a variety of bacteria that thrive in environments with little or no oxygen. The steps of denitrification include:
- Nitrate Reduction: Denitrifying bacteria first reduce nitrate to nitrite (NO2-), an intermediate step in the conversion back to nitrogen gas.
- Further Reduction: Nitrite is then further reduced to nitric oxide (NO), nitrous oxide (N2O), and finally to nitrogen gas (N2), which is released into the atmosphere.
This process is essential for maintaining the balance of nitrogen in aquatic environments. Without denitrification, nitrates could accumulate to harmful levels, leading to eutrophication and the degradation of water quality. Denitrification has several environmental implications:
- Prevents Excessive Algal Growth: By removing nitrates, denitrification helps prevent the overgrowth of algae that can lead to harmful algal blooms.
- Water Quality Maintenance: It plays a significant role in water purification processes, especially in wetlands and other natural water filtration systems.
- Greenhouse Gas Emissions: While beneficial for removing nitrates, denitrification can produce nitrous oxide (N2O), a potent greenhouse gas. This highlights the importance of managing denitrification processes to minimize environmental impact.
Understanding denitrification is vital for the effective management and conservation of aquatic ecosystems, ensuring they remain healthy and vibrant for future generations.
Human Impacts on the Nitrogen Cycle
Human activities have significantly altered the natural nitrogen cycle, with profound effects on ecosystems and the environment. These impacts stem from various sources and have both positive and negative consequences:
- Agricultural Practices: The use of synthetic fertilizers has greatly increased the amount of biologically available nitrogen in soil and water. While this has boosted crop yields, it has also led to nutrient runoff into water bodies, contributing to eutrophication and the formation of dead zones in aquatic ecosystems.
- Industrial Activities: The combustion of fossil fuels for energy and transportation releases various nitrogen oxides (NOx) into the atmosphere. These pollutants contribute to the formation of smog and acid rain, which can harm aquatic life and damage ecosystems.
- Wastewater Discharge: Effluent from sewage treatment plants and industrial facilities often contains high levels of nitrogen. When discharged into water bodies, it can exacerbate eutrophication and negatively affect water quality.
- Land Use Changes: Deforestation, urbanization, and the conversion of natural landscapes into agricultural land can disrupt local nitrogen cycles. These changes reduce the soil"s ability to naturally process and cycle nitrogen, leading to increased nitrogen loss to the atmosphere and waterways.
The cumulative effect of these human activities on the nitrogen cycle is substantial, affecting biodiversity, water quality, climate change, and human health. Efforts to mitigate these impacts include improving agricultural practices, reducing industrial emissions, enhancing wastewater treatment processes, and restoring natural habitats that can effectively cycle and store nitrogen.

Effects of Nitrogen on Aquatic Life
Nitrogen, while essential for the growth and development of aquatic life, can have varied effects on water bodies, depending on its concentration and form. Here’s how nitrogen impacts aquatic ecosystems:
- Supports Growth: Nitrogen is a key nutrient that supports the growth of aquatic plants and phytoplankton. It is crucial for photosynthesis and the production of amino acids, nucleic acids, and proteins.
- Algal Blooms: Excessive nitrogen, often from agricultural runoff or sewage discharge, can lead to algal blooms. While some algae are beneficial, blooms can deplete oxygen levels in water, creating hypoxic conditions harmful to fish and other aquatic organisms.
- Impact on Water Quality: High nitrogen levels can affect the quality of drinking water. For instance, nitrate, a common form of nitrogen in fertilizers, can contaminate groundwater and pose health risks to humans and livestock when ingested at high levels.
- Biodiversity Loss: The imbalance in nutrient levels can lead to a loss of biodiversity. Eutrophic conditions, spurred by excessive nitrogen, can cause a decline in fish populations and other aquatic species, as it alters the natural balance of aquatic ecosystems.
- Toxicity to Aquatic Life: Ammonia, a form of nitrogen waste produced by fish and decomposing organic matter, can become toxic to aquatic organisms at high concentrations, affecting their growth, reproduction, and survival.
Managing nitrogen inputs into aquatic systems is vital for preserving water quality and protecting the health and diversity of aquatic life. Strategies such as improved wastewater treatment, sustainable agricultural practices, and restoration of natural wetlands play a critical role in mitigating the adverse effects of nitrogen on aquatic ecosystems.
READ MORE:
Managing Nitrogen Levels in Aquatic Ecosystems
Effective management of nitrogen levels is critical for maintaining the health of aquatic ecosystems and ensuring the sustainability of water resources. Here are several strategies that can be employed:
- Reduce Agricultural Runoff: Implementing best management practices in agriculture, such as precision fertilization, crop rotation, and the use of cover crops, can significantly reduce nitrogen runoff into water bodies.
- Enhance Wastewater Treatment: Upgrading wastewater treatment facilities to include advanced nitrogen removal processes helps reduce the amount of nitrogen entering aquatic ecosystems from municipal and industrial sources.
- Restore Wetlands: Wetlands act as natural filters, removing nitrogen from water through plant uptake and microbial processes. Restoring and protecting wetland areas can enhance their capacity to mitigate nitrogen pollution.
- Regulate Industrial Emissions: Enforcing stricter regulations on industries to control emissions of nitrogen oxides (NOx) can reduce the amount of nitrogen deposited in water bodies from the atmosphere.
- Public Education and Awareness: Raising public awareness about the sources and impacts of nitrogen pollution can encourage community involvement in reducing personal and collective nitrogen footprints.
- Support Sustainable Farming and Land Use: Encouraging sustainable farming practices and responsible land use planning can prevent excess nitrogen from entering aquatic systems.
By integrating these strategies, communities and policymakers can work towards a more balanced nitrogen cycle, protecting aquatic life, preserving water quality, and ensuring the resilience of aquatic ecosystems against the challenges of nitrogen pollution.
Exploring the nitrogen cycle in aquatic ecosystems unveils the intricate balance of nature, highlighting our role in safeguarding water"s vitality for future generations. Embrace the journey towards understanding and protecting this essential life process.